Products In Development
Our Products Still Under Clinical Development
PRODUCTS IN CLINICAL DEVELOPMENT
Matrix III® Cisplatin
Cisplatin Resorbable Sustained Release Implant
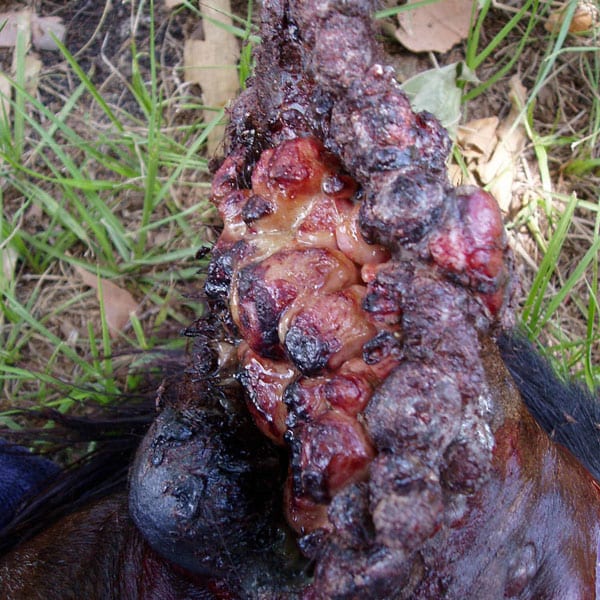
Indication: Treatment of Equine Sarcoids (MUMS – Minor Use Minor Species)
Product Description: Cisplatin Resorbable Sustained Release Implant is comprised of a proprietary combination of Calcium Sulfate Hemihydrate USP, Dextran Sulfate Sodium Salt and Cisplatin USP. Each ~3mm bead contains 1.6mg of Cisplatin that is slowly released at the site of implantation over a period of 7-10 days.
Regulatory Status: Active Investigational New Animal Drug () in the US. The USFDA has designated the beads as MUMS, a minor use for horses with sarcoids. The Targeted Animal Safety Study (TASS) has been completed in support of the indication being pursued: “the local treatment of sarcoids up to 3 cm in diameter, following surgical debulking via sharp excision excluding lesions that are flat or those that are located within 1.5 cm of a vital anatomical structure, such as the eye, urethra, or rectum in horses.”

*Slide Arrows Left of Right to see Full Image
Clinical Experience: Hundreds of horses and dogs have been treated with Royer’s Cisplatin Resorbable Sustained Release Implant, treating a variety of Cutaneous Neoplasias with impressive clinical outcomes.
Before Treatment After Treatment
“I just wanted to share this result with you. I never thought this ear would be saved. Today was the fourth time we’ve implanted beads, but as you can see, the results are pretty dramatic. I am impressed and the owner is absolutely thrilled. I’m sure the horse feels better too! I love the safety and ease of working with your product. Thanks!”Equine veterinarian in Florida
Current Clinical Trial(s)
A clinical trial is currently being conducted at multiple sites across the US in support of the desired indication:
“the local treatment of sarcoids up to 3 cm in diameter, following surgical debulking via sharp excision excluding lesions that are flat or those that are located within 1.5 cm of a vital anatomical structure, such as the eye, urethra, or rectum in horses.”
Upon completion of the trial, targeted for 2020, a “Reasonable Expectation of Effectiveness” (RXE) will be submitted to the Center for Veterinary Medicine at the FDA in support of Royer Biomedical’s rolling New Animal Drug Application.
In the interim, implantable cisplatin beads using patented Matrix III™ drug-delivery technology will be available through a compounding pharmacy under an exclusive license from Royer Biomedical. (Coming soon)

